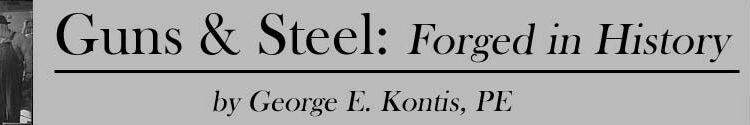By George E. Kontis, PE
When man first walked the planet, metal ores of iron could be found just lying about. The ancients eventually figured out how to extract it and form useful items from iron – the fourth most plentiful element on the planet. As early as 2000 BC, bronze began to be replaced by metallic iron which was extracted from ore though a smelting process. A hot charcoal fire was necessary in order for the oxygen in the iron to combine with the carbon monoxide in the ore to form carbon dioxide, leaving the molten metal behind. The iron was produced along with a lot of impurities known as slag. For many years, blacksmiths would remove the iron from the furnace and hammer it on an anvil to drive out the cinders and slag. Forged this way, this “wrought iron” could be worked to remove a large percentage of the carbon. Wrought iron is tough and easily formed, and for that reason it found widespread use in tools and weapons.

At very high temperatures the high carbon content, 3 to 4.5%, in cast iron, makes it extremely hard and durable. Because of the extreme hardness it is not easy to forge. Wrought iron has between 0.02 to 0.08% carbon, which makes it malleable and ductile, but not incredibly strong or hard. Just in between these two, with a range of 0.2 to 1.5 %, steel has properties of both of the irons but can be made harder, more ductile, and malleable.
Steel was known to be the better and more useful form of iron but its manufacture was not widely known. The Hittite tribes and sword makers in India kept the secret of steel making as their own. Since steel has more carbon content than wrought iron, it was usually made by accident, when the blacksmith left a hot piece of wrought iron in the hot charcoal overnight. This introduced more carbon into the wrought iron, making steel. One thing was certain, no matter by what means it was made, production of steel was slow and painstaking. Rarely was more than a small quantity produced at one time. When the first hand cannons showed up in the 1200s the material choices were limited to bronze, wrought iron, or cast iron.

In the late 1700s a method called “puddling” was used to separate the more desirable, wrought iron from the molten iron. A special furnace, developed by an Englishman named Henry Cort, was designed so a skilled craftsman, called a “puddler” could stir the molten metal in a special part of the furnace. As the carbon content decreased, the melting point would rise, so small bits of iron would appear in the liquid mass. The job of the puddler was to observe when the solid pieces would occur, gather them, and work them in a forge. Unfortunately, the process was painfully slow. The demand for wrought iron was high and the well-paid puddlers were unable to keep up. Mechanization of the process was tried many times but always without huge success.
For most of the 1700s the British were the best at manufacturing steel. So envied was their expertise that Napoleon offered a sum of 4,000 francs to anyone who could match the British process. A young German, Friedrich Krupp, was fixated on this prize. So much so, that even without a solid scientific background, he opened Gusstahlfabrik – Cast Steel Works – in Essen, Germany on 20 September, 1811. Its purpose was “for the manufacture of English cast steel and all articles made thereof.” Krupp failed miserably and died at 39 with little success in steel making and a mountain of debt. After a very brief mourning period, his energetic 14 year old son, Alfred, walked into the mill, determined to learn how to make the world’s best steel. At first, it was not of English quality, but was consistent and good nonetheless. His primary “secret” was getting a high grade ore from Sweden. Swedish ore had very low phosphorus content, an element that made steel brittle. Alfred was continuously looking for ways to improve his process and thought he might get a good head start in England. He spent five months there trying to figure out what they were doing but came away with more questions than answers.

Undaunted, Krupp kept his focus, developed his own technology, and produced two hollow-forged, cold drawn steel musket barrels in 1843. Alfred went immediately to the Prussian military to see if they were interested, but the officer on duty thought the idea of a steel weapon actually funny. The Prussians considered them briefly, but returned them with little enthusiasm. Krupp went to the English and the Germans, but nobody was interested. Next he tried building a steel barreled artillery piece. The Prussians tested it and liked it, but it was too expensive and they had no requirement, preferring to stay with the bronze guns that worked so well for them at Waterloo.
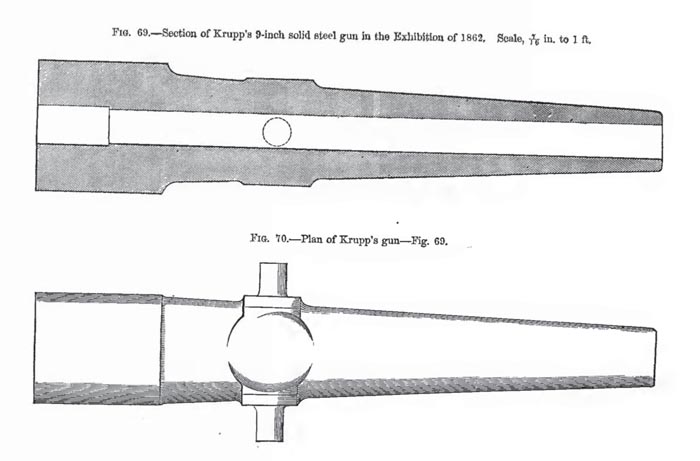
At the very first World’s Fair held in 1851 at the Crystal Palace in England, Krupp decided to make his big move. It was time for some unprecedented showmanship. For his display, he brought a 4,300 pound steel ingot – twice as large as anything coming out of England. There was one more display item: Highly polished and beautifully crafted with beech wood trim, it was a thin-tubed breech loading steel cannon. As he predicted, it was the cannon and not the ingot that got all of the attention. At that time it was the largest cannon ever forged without welds. Krupp thought it might have too much new innovation for his old fashioned potential customers so he was careful to point out that the breech plug could be secured and loading could take place from the muzzle if desired.
The English press had a field day writing pros and cons about Krupp’s cannon. Most doubted that the thin walls would resist the high pressure of firing successive charges, but everyone marveled at the beautiful workmanship. It was magnificent. Like a proud father, Krupp came prepared for questions about his shiny steel baby. With every price list, Krupp handed out a four page technical document, presenting the calculations of Dr. H. Scheffler. Recognized in Europe for his work in structural analysis, Dr. Scheffler had recently written a book validating the Lamé equations for calculating stresses in thick walled cylinders. These same equations, formulated by the famous French mathematician, are the same ones gun barrel designers use today. Scheffler used the Lamé equations in an example that compared three gun tubes of equal proportions. He showed that the cast iron barrel would burst when the chamber pressure exceeded 18,000 pounds per square inch (psi), the bronze barrel at 33,000 psi, and the steel barrel at 117,000 psi.
Krupp received many requests for loans and sales from world leaders and royalty. The end results were disappointing. In Russia, military evaluators fired 4,000 rounds in an exhaustive test without as much as a scratch in the bore – a feat that far exceeded the capability of their bronze cannons. They were amazed, but not ready to buy. What they thought they had witnessed was a miracle. It was one of a kind to be enshrined forever in the Museum of the Fortress of Peter and Paul – which they did, at the same time neglecting to pay Krupp.
Krupp was the pioneer who determined that steel is the ideal form of iron for firearms including small arms, cannons, and other weapons. He realized that the high hardness, flexibility, strength, and wear resistance was ideal for firearms. Yet with all these advantages, steel remained difficult to produce in the quantity and quality needed. The demands for steel kept growing, but production remained slow. The culprit was carbon. There was just no good way to regulate it.
Finally, a better steel making method did emerge. The process involved shooting a blast of hot air up through the molten iron. This caused the carbon monoxide to combine with the oxygen, driving off much of the carbon to make steel. Many of the impurities were burned off at the same time. It was called the Bessemer process, after the Englishman who invented it. Before his work in steel production, Bessemer was an inventor of some renown who, by his own admission, knew little about metallurgy. In 1853, the Crimean war had united the French and British forces against Russia and inspired many inventors to focus their interest in advancing the state of the art in weaponry. Henry Bessemer was among them. He had received a patent on a grooved projectile, designed to fire in smooth bore weapons. The grooves were intended to act like a wind turbine to spin the projectile enough to stabilize it. The projectiles were long and heavy which caused the chamber pressure to rise to dangerous levels during firing. Most of the development took place in France with Bessemer working alongside Commandant Claude-Etienne Minié, who was famous for the development of a rifle bearing his name.

Here is a short version of the steel process invention story as Bessemer related in his autobiography: One December day, just before Christmas, Bessemer spent the day attending shooting trials in Vincennes. When the trials ended, Bessemer caught a ride back to Paris in a horse drawn carriage. On the ride he reflected on a conversation with Commandant Minié who told Bessemer that he was afraid that unless a better material came along soon, all of their development work would be for naught. Bessemer said this “was the spark which kindled one of the great revolutions that the present century has to record, for during my solitary ride in a cab that night from Vincennes to Paris, I made up my mind to try what I could to improve the quality of iron in the manufacture of guns. At that time nearly all our guns were simply unwrought masses of cast iron, and it was consequently to the improvement of cast iron that I first directed my attention. My knowledge of metallurgy was at this time very limited but this was in one sense a great advantage to me, for I had very little to unlearn and could let my imagination have full scope.” Bessemer said he went on to develop the air blowing process, obtaining a U.S Patent in 1854.
Bessemer pocketed huge royalties from licensing out his technology, and was knighted in 1879 for his contribution to science. His account of his contribution to the world’s first high volume steel making process did not play well in Pittsburgh, Pennsylvania. It seems another story about a home town boy, a pretty girl and a very pesky mineral called shadrac has more credulity there. William Kelly studied metallurgy at the University of Pittsburgh, and after graduation went to work in his father’s dry goods business. While on a sales trip to Nashville, he met Mildred Gracy, arguably the most beautiful 16-year old girl in her high school class. Inspired, Kelly moved to her home town, Eddyville, Kentucky, where he opened a general store. The business was a success, Kelly was accepted by her parents, and Millie became his bride.

At heart, Kelly was a metallurgist, so when he found high grade iron ore lying above ground, near the banks of the Cumberland River near his home, he could not contain his excitement. What an opportunity! Besides the iron ore, there were plenty of trees for fuel, vacant land, and a river for transportation. He and his brother bought 14,000 acres and began producing cast iron products. When the surface ore ran out, they found the subsurface ore to be of a much lower quality, containing a form of flint called shadrac. When they tried to smelt the ore they found they could not rid the molten metal of shadrac.
Kelly had a very good understanding of chemistry and metallurgy, and theorized that blowing air up through the molten steel might cause the carbon in the steel to combine with the oxygen, burning off impurities, and freeing the steel from the excessive carbon. Almost everyone he discussed it with, thought he was crazy – surely the molten metal would get cooler not hotter when the cold air passed through it! It didn’t help that he told everyone his new process was going to “make steel without fuel.” Kelly’s theory became an obsession that almost wrecked his business and his marriage, but he persisted until his “pneumatic process” of refining iron was proven out.
Always on the lookout for hard workers, Kelly was pleased when two energetic and inquisitive Englishmen showed up looking for work. Up to this point, Kelly had decided to keep his process secret, and the two Englishmen seemed so honest and were so helpful and hardworking that he trusted them as he did all of his workers. It was only when the two Englishmen left suddenly and without warning that Kelly became concerned. Worried his secret might be compromised, Kelly tracked them to a ship that had left for England. It was not long afterwards that Bessemer filed for a U.S. patent to protect “his” process. At that point in time, Kelly had already completed his experiments and had been producing steel with his pneumatic process for about seven years. Kelly challenged Bessemer in U.S. courts, who found in favor of Kelly and granted him a patent that was for some time, an impediment to the Bessemer claims.
While the Kelly and Bessemer process was effective in removing carbon and other impurities from molten iron, it was not a process that was easy to control. Their processes worked so well, that it often removed too much carbon and instead of producing steel, it made wrought iron. Neither steelmaker could figure out how to stop their process at the right time. Along came an English metallurgist named Robert Mushet, who came up with an amazingly simple solution. That was to continue the blasts of air and operate the process until most of the carbon was gone. At that point, carbon was put back in. With the amount of molten iron known, the exact amount of carbon could be added to yield steel with just the right percentage of carbon.
The Bessemer process aided by the Mushet development boosted the production level of steel around the industrialized world, but there were limitations. There was still a high level of sulfur and phosphorus in the steel, and the phosphorus was particularly bad since it made the steel brittle. That problem was solved by restricting the Bessemer process to use only expensive, high grade ores from Sweden and Wales which had naturally low phosphorus content.
In spite of the flurry of activity circulating around the new and easier method of making steel, the introduction of steel into U.S. weapon development was slowed because of the focus on the Civil War that began in 1861. There is another interesting reason: The U.S.-produced iron used in cannon making was much better than the best irons the English could produce and the U.S. designs were excellent. Two Union officers, Army Captain Rodman and Navy Admiral Dahlgren contributed to this success. Both of them were innovators, pushing the state of the art in casting techniques and cannon design. Admiral Dahlgren found ways of combining cast iron, wrought iron, and bronze to achieve the best performance at a safe level. Some of Dahlgren’s cannons were a unique bottle shape that usually went 1,500 to 2,000 rounds before cracking. Oddly, in spite of their shape, the Rodman guns, the Dahlgren guns, and all the others eventually broke, and all in the same manner – split down the middle at the breech with a crack that went forward past the trunnions, then travelled sideways in opposite directions, leaving three large, useless chunks.
Designers sometimes went to the extreme in reinforcing their cannon barrels. Take for example the cannon designed by Lynall Thomas in 1862. His 7-inch gun was made from rolling a 1-inch plate of wrought iron around a cast iron steel tube. Two 13-inch bands of wrought iron, 3 inches thick were wrapped around the breech for reinforcement. This 11.5 foot long beast burst on the second round after being test fired.

When the Civil War was over, in 1865, Dahlgren and Rodman proclaimed that wrought iron was just too soft and too weak for the production of guns. It was time to turn to steel. Formed to create industry standards, the American Iron Institute had just changed its name to American Institute of Iron and Steel (AISI,) after being established in 1855. The AISI even modified their charter to include the organization and standardization of steel alloys. By then it was realized that higher the carbon, the stronger the steel, so one of the AISI’s first jobs was to classify carbon steels. Low carbon steels were defined to have carbon in the range of 0.08 to 0.15%, medium 0.15 to 0.35%, and high carbon steels 0.65 to 1.2%.
Improvements in steel and steel making came fast in the second half of the 1800s. In 1875, Sidney Gilchrist Thomas, discovered that burned limestone added to the molten iron was effective in removing phosphorus from iron ore. This meant the Kelly/Bessemer process could use even low quality ores for steel production.
In the beginning of the 1800s chemists discovered and isolated other metals that weren’t of much use by themselves but now found they could be alloyed with steel to improve its properties. Robert Muchet discovered that alloying with vanadium would produce better steel – up to three times the strength of common steel. Manganese was added to improve workability, strength, and wear resistance. Nickel and chromium imparted toughness, heat and corrosion resistance, while molybdenum offered hardenability and heat resistance. At the dawn of the 20th century, cast and wrought iron firearms were quickly becoming a distant memory. Time has diminished the interwoven history of steel and guns as these and other steel alloys ushered in a new era in weapon design.
| This article first appeared in Small Arms Review V16N3 (September 2012) |