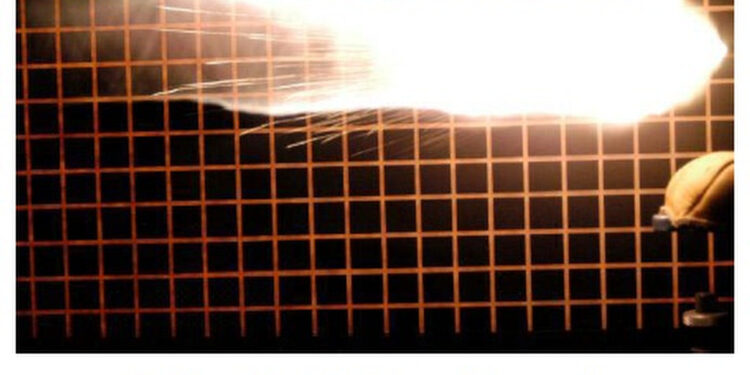
The problem of ammunition deteriorating and failing to fire seems to have become bigger in recent years with relatively new ammo failing to fire in some instances while other, much older, ammo remains sure fire. I have experienced this problem firsthand losing a fine 8-point buck when a new factory cartridge would not fire. I have heard some people say they think the government is behind this in order to prevent folks from stockpiling ammo. I don’t know about that. What I do know is the cause and the cure and that’s what this article is about.
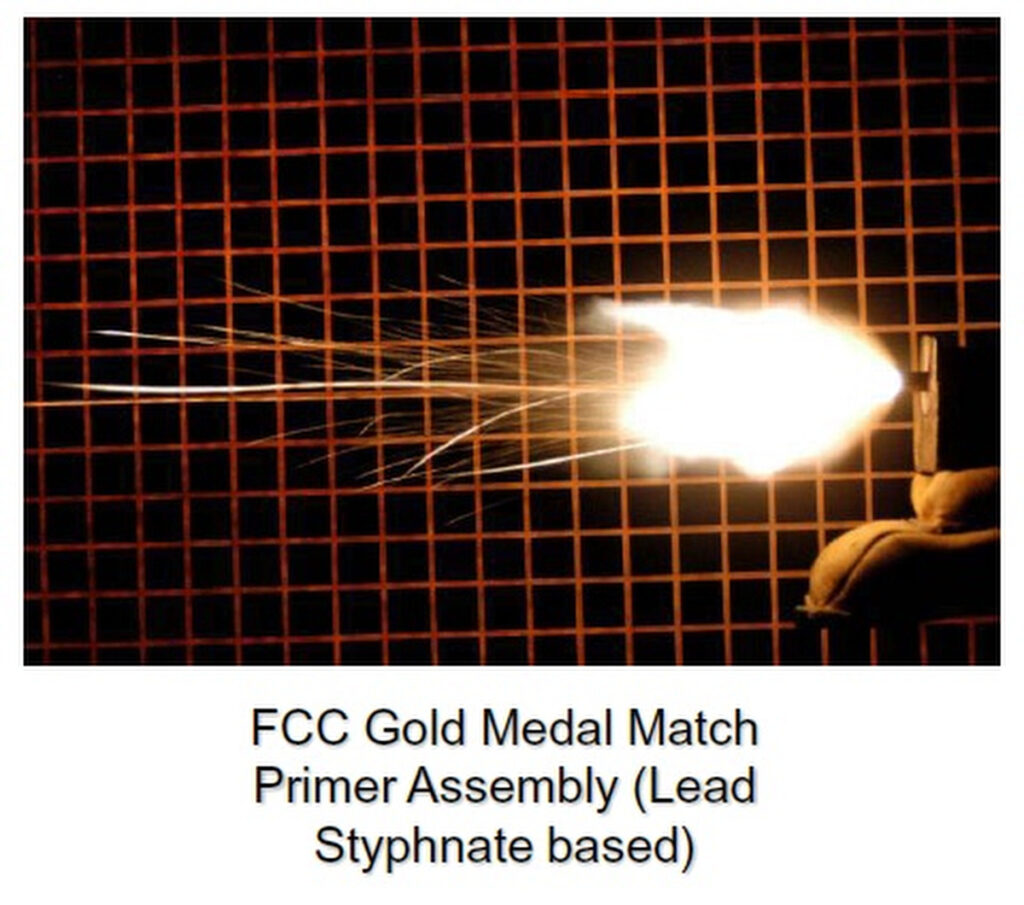
John Flanagan of Sabot Designs, a manufacturer known for his flechette-loaded shotgun shells, pointed out that the usual culprit was solvent in the powder doing what solvents do, reacting with other chemical compounds, in this case the primer, rendering the round a dud. As a contractor making ammunition for the government, Flanagan is well aware of this problem and how to deal with it. Flanagan also helped me get the relevant government documents I’ll reference in this story.
THE SOLVENT ISSUE
To begin with, nitrocellulose powders require a solvent to gelatinize them so that they can be formed into balls, flakes, cylinders, or strips. Single base powders are typically an ether-alcohol colloid of nitrocellulose. Double base powders are made from nitrocellulose and nitroglycerine and use ether, ethyl acetate, or isooctanol, as processing solvents. Much of this solvent is lost in processing. The solvent must remain at the minimum level to keep the propellant from becoming brittle and losing plasticization causing the powder grains to turn to dust which will raise pressure dangerously as the smaller the fuel the faster it burns. Just look at how much faster a pile of wood shavings burns than a big log does. Without a certain amount of solvent, you cannot keep the dimensional stability of the powder grains.
This solvent will also act on primer compounds, rendering them incapable of detonation. Because of this, the mil-spec limit for residual solvent in the dried powder is 0.25 weight-% (0.25% of the powder weight). If this limit is exceeded, the life of the primer will be shortened accordingly. These solvents off-gas from the powder and are what you smell when you open a powder can. Back in the days of the Army Coast Artillery, the powder magazines contained so much ether that many thought it was put there deliberately to somehow preserve the powder when, in reality, it was just massive amounts of off-gassing in a confined area.
STUDIES HAVE BEEN DONE
To give you an idea of how much gas can come out of the powder, here is the results of a U.S. military test. A 75-liter drum was filled with 150 pounds of propellant powder containing residual solvent content of 0.25 weight-% (the mil-spec limit for dried propellant). There were approximately 70 grams of solvent. 12 grams of solvent were in the vapor phase and of these, 7 grams were in the space above the propellant and 5 grams occupied the space between the powder grains. The remaining 158 grams of solvent were trapped in the propellant grains.
The 12 grams in the vapor phase were in the form that can act as a solvent on the primer compound. When you add a solvent to primer compound you interfere with its performance, as you’re breaking down the explosive mixture.
This information came from a several government studies I found including:
- 1951, Great Britain’s Ministry of Defense study, Interior Ballistics authored by F.R.W. Hunt (pages 1-7)
- 1966, Julian S. Hatcher, Hatchers Notebook (pages 353-360)
- 1970, E.R. Lake’s Percussion Primer Design Requirements. (pages 2-5)
- 1994, U.S. Army Research Laboratory LOVA Propellant Aging: Effects of Residual Solvent study (pages 1-2, 6, 11, 13)
- 1980, U.S. Army Armament Research and Development Command study titled A Compilation of Hazard and Test Data for Pyrotechnic Compositions (pages 57, 62, 63.)
- 2019, Joint Ordnance Test Procedure (JOPT)-022 Safety and Suitability for Service Assessment Testing for Small Caliber Ammunition Less Than 20MM (appendix A.1-A.5)
WHY IT HAPPENS
Everyone knows not to store ammo at high temperatures, but few know the real reason why. High temperatures drive the solvent into the vapor phase and the solvent vapor kills the primers.
Nitrocellulose itself deteriorates over time yielding acidic byproducts such as nitric acid which can also attack primers. Some powders contain calcium carbonate to neutralize these acids. To absorb the decomposition products of nitrocellulose and nitroglycerine-based powders and prevent their buildup and catalyzing the decomposition stabilizers are added such as diphenylamine, mineral jelly, carbamite, pictrite, and calcium carbonate. These may also act as cooling agents and gelatinizers. These stabilizers can be a health hazard in large quantities and the military has been doing studies on their effect on artillerymen who encounter massive amounts of smoke and chemical residue when firing their artillery.
The combination of all these solvents, acids, and chemicals reacting together results in a complicated combination of solvent gasses with multiple compounds present to interact with your primers. Even the oxidation of lead bullets can spread to the primer and result in a dead primer.
Among the escaping solvent gasses are ether, ethyl acetate, acetic acid, oxalic acid, nitric acid, carboxlic acid, isocatanol, and isoctanoic acid. Ingredients may vary due to the different chemicals initially present in the powder.
All of this volatile chemical cocktail residing in a cartridge case is injurious to primers and over time, when stored improperly, can turn them into duds. If the powder contains more residual solvent content than the mil-spec previously alluded to then the primer will go bad much faster. This seems to be the case with some of the recent lots of commercial ammo that has quickly gone bad in 10 years or less.
From a manufacturing perspective, it’s clear that too much solvent will kill the primer. J. J. Reich of Federal Ammunition says that this is, indeed, why high heat will cause ammunition to go bad. The heat drives the solvent out of the powder and sets it loose to attack the primer. Reich also pointed out that some of the current powders (not those used by Federal) are so hydroscopic that they will absorb enough water in a normal humidity house for the powder fail to ignite.
Joel Hogdon of Remington Ammunition says his company avoids this issue by storing its powder where ventilation can prevent the buildup of gasses coming off the powder and spoiling it by sticking around. He advised that any powder that is discolored, causing a powder can to bulge, or producing a bad smell, should be discarded. Primers, he said, should not be unboxed and left out to absorb moisture before loading into cartridges.
Interestingly, some bullets are now made of porous, powdered metal that can admit moisture to the powder within the cartridge case. The solution to this is a liquid sealant, says Jonathan Langenfeld, the head of R&D engineering at Remington Ammunition. But some types of modern primers must be able to breathe, so you apply the sealant to one side of the primer on some and both sides on others… as well as the bullet. Vacuum seal-finished ammo is another a surefire way to beat moisture absorption by certain hydroscopic gun powders and primer compounds.
OTHER CAUSES
There is one other compound that can sometimes attack primers. Years ago, I had some lead-bullet 38 S&W ammo go bad while the Winchester Lubaloy-coated bullets of the same vintage all fired. The Montana state chemist at the time, John Buchanan, told me that sometimes lead oxide from old lead bullets can react with primers and kill them.
Another thing to consider is that primers require a fast, hard strike penetrating a minimum of 0.017-inch depth to go off. Seth Swerczek at Hornady Ammunitionpoints out that if improper headspace puts the primer out of reach, or there is a slow lock time as can occur with dirt or with congealed lubricant in cold weather, then the primer may not be indented fast enough to fire. He also said that it was sometimes possible for 1-3 primers out of a million produced to be incomplete, lacking anvil or primer compound and thus incapable of firing, though he stressed this was very rare.
THE SOLUTION
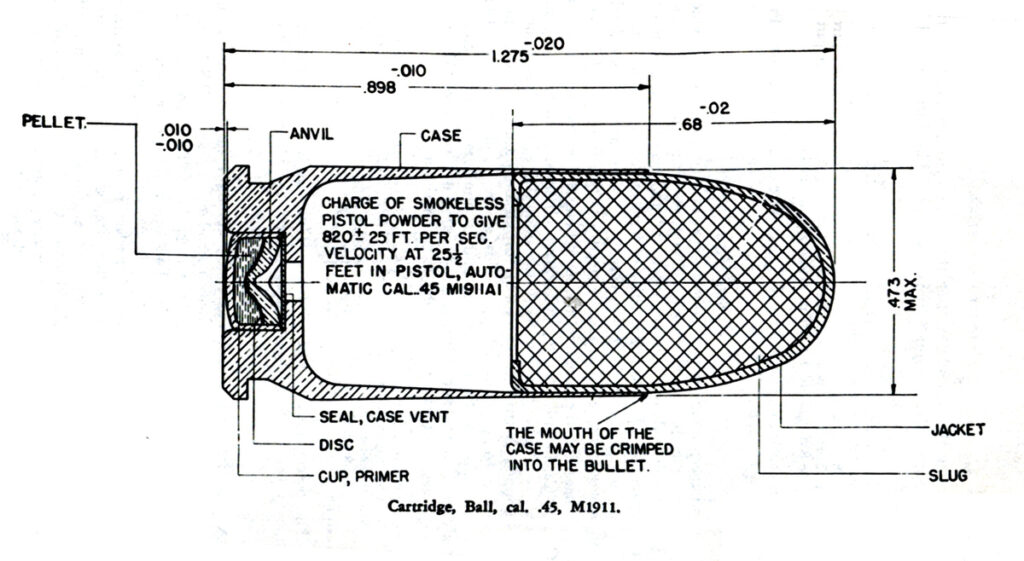
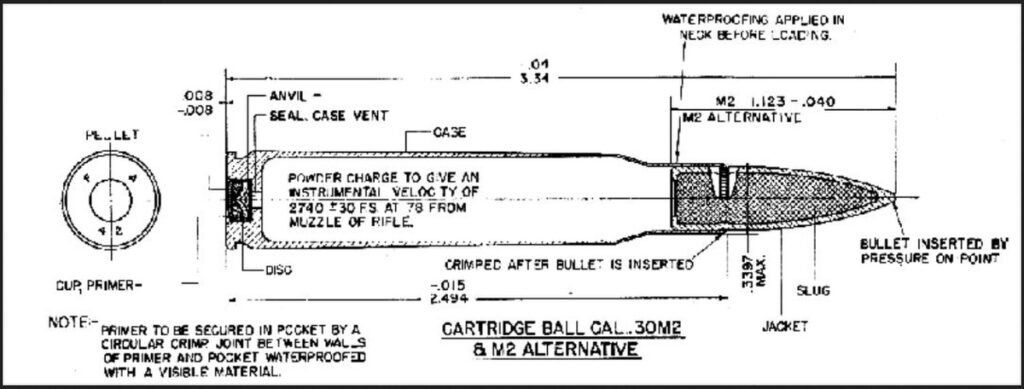
The solution is two-fold, depending on how you get your ammo.
For handloaders, use powder that does not exceed the mil-spec limit for solvent in dried propellant. Then, when making your cartridges, shield the primer from the solvents and acids. This was done in the past by the military as shown in the Army’s production drawings of the .45 ACP and 30-06 ammo. Note the part labeled “Seal, case vent” between the primer and the powder charge. That seal is a ten thousandth of an inch thick celluloid plug. It was installed by inserting a strip of ten thousandth of an inch thick celluloid over the primer pocket before seating the primer. The primer punches out the celluloid plug when it is installed, and it stays in place. Ammo thus sealed does not go bad. This is a very cheap and simple solution that anyone loading ammunition can employ regardless of whether you have a big factory with giant plate loaders turning out millions of rounds or a simple Lyman tong tool for reloading.



Handloaders can source these celluloid strips from Flannigan at Sabot Designs LLC, who I previously mentioned above. Flannigan has agreed to sell these strips to any individual handloader or ammunition manufacturer that wants them. Once in place, the primer is protected from the solvents and acids from the propellant powder, so things always go bang when they are supposed to.
For those buying commercially loaded ammunition, look for fresh ammunition lots and store your ammunition away from heat, humidity, and gun cleaning solvents, as these solvents can also attack and kill primers, especially those solvents with an ammonium base.
In the past people blamed the primers but blaming the primer after chemically attacking it is not right. You must put the blame where it belongs. On the chemicals that neutralized the primer.